Keywords
|
| Janus green B, Ammonium phosphomolybdate, Photocatalytic degradation. |
INTRODUCTION
|
| Most of the aquatic systems are under severe threat of pollution due to the ever increasing industrialization, urbanization, and population exodus. Various pollutants like acids, alkalies, detergents, soap, phenols, cyanides, metals, pesticides, insecticides, fungicides etc., released from different chemical industries pollute our water resources. Pollution is also caused by industrial wastes of leather tanneries, textile, dyeing, printing, metal extraction distilleries, oil refineries and pharmaceuticals units. The excess assimilation of these wastes in water resources will pollute the whole water and will create many side effects. |
| The commonly used waste water treatment process include absorption, oxidation, biological methods, etc.The photocatalytic degradation of organic pollutants is having a direct relevance in water remediation treatments. Semiconductor are used to degrade organic pollutants in water to less harmful inorganic material was observed by Li et al. (2005). Photocatalytic degradation of waste water pollutants and surfactants by TiO2 was investigated by Muneer et al. (1992). Photoreduction of methyl orange using TiO2 as photocatalyst was observed by Brown and Darwent (1984). Photocatalytic degradation of ethyl violet in aqueous solution with TiO2 suspension was reported by Chen et al. (2006). The kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor was observed by Mahmoodi et al. (2006). Photodegradation of methylene blue using solar light and TiO2 (as semiconductor) was investigated by Nagueria and Jordin (1993). The use of ZnO particulate system as a photocatalyst in photobleaching of xylidine ponceau and orange G was observed by Sharma et al. (1995). Photocatalytic degradation of brilliant green over semiconductor ZnO powder suspended in aqueous solution has been reported by Ameta et al. (1997, 1999). |
| Neppolian et al. (1999) reported Photodegradation of some textile dyes over ZnO. Mansoori et al. (2004) observed photobleaching of rhodamine-B over ZnO. Evaluation of Nb2O5 and Ag/Nb2O5 in the photocatalytic degradation of dyes from textile industries has been investigated by Silva et al. (2002). Chu and Tsui (2002) observed photodecoloration of a non-biodegradable azo dye, reactive red 2, in a mixture of acetone and triethylamine solution. |
| Although a lot of work has been carried out in the field of photocatalysis using TiO 2, ZnO, CdS, ZnS etc. but negligible attention has been paid to ammonium phosphomolybdate as a semiconductor. Ammonium phosphomolybdate is yellow in colour and absorbs visible radiations. Ammonium phosphomolybdate serves as an excellent candidate for photocatalysis, because of its optical properties, which include a high refractive index, chemical stability, low cost, ready availability, greater efficiency, selectivity and convenient way of treating several undesirable chemicals. It has been used as a photocatalyst for the reduction of some metal ions in low and as well as high concentrations, but it has not been used for the degradation of dyes so far. Therefore, it has been planned to use ammonium phosphomolybdate for photocatalytic degradation of some dyes. Janus green B has been selected for the present investigation. |
| Janus green B belongs to azo group of dye, it is a basic and cationic dye used in histology and for mitrochondria supravitally (1900). Janus green B undergoes significant degradation in the presence of ammonium phosphomolybdate semiconductor under visible light irradiation. The ammonium phosphomolybdate -assisted photodegradation of dyes (pollutants) is an interfacial reaction, which occurs on the surface of semiconductor. Therefore, the dye adsorption on the semiconductor surface plays an essential role in the photodegradation process. |
| The degradation rate of dye was found to be a function of various parameters such as concentration of janus green B, amount of semiconductor, light intensity, and pH. The main goal of the present study is to investigate the photocatalytic activity of ammonium phosphomolybdate semiconductor for degradation of janus green B. |
EXPERIMENTAL DETAILS
|
MATERIAL
|
| Janus green B (Loba chemie) and ammonium phosphomolybdate (Himedia) were used in present investigations. |
Appartus
|
| Digital pH meter - The pH of solution was measured by a digital pH meter (Systronics Model-371). |
| Solarimeter - The intensity of light was measured by solarimeter (CEL Model-SM 201) in mWcm-2. |
| Light source - A 200 watt tungsten lamp was used for irradiation purpose. |
| Spectrophotometer - Optical density (O.D.) was measured at different time intervals by U.V.-visible spectrophotometer (Systronics Model -104). The colour fading of the dye was monitored spectrophotometrically at 615 nm. G-3 sintered glass crucible was used for filtration to obtain the desired accuracy in the measurement of optical density of dye solutions. |
Procedure
|
| 0.0511 g of janus green B was dissolved in 1000 mL of doubly distilled water, so that the concentration of dye solution was 1.00 × 10–4 M and it was used as a stock solution. This solution was further diluted. The optical density of this dye solution was determined with the help of a spectrophotometer (λmax = 615 nm). 200 mL. of dye solution was taken and divided into 50 mL. of this dye solution was taken in four beakers, each. |
| 1) The first beaker containing only janus green B so lution was kept in dark. |
| 2) The second beaker containing only janus green B solution was kept in light. |
| 3) 0.20 g ammonium phosphomolybdate semiconductor was added to third beaker containing janus green B solution and was kept in dark. |
| 4) 0.20 g ammonium phosphomolybdate semiconduc tor was added to third beaker containing janus green B solution and was kept in light. |
| These beakers were kept for 4 hours. It was observed that solution of first three beakers had the same optical density and colour, while the solution of fourth beaker showed a decrease in its initial value of optical density. |
| The above experiment confirms that the reaction between janus green B and ammonium phosphomolybdate is not chemical and photochemical reaction but it is a photocatalytic reaction. |
| Photocatalytic degradation of janus green B was studied by taking 50 mL of 8.4 × 10-5 M solution in a 100 mL beaker and the pH = 10.5 of the dye solution was adjusted with the help of previously standardized sulphuric acid and sodium hydroxide and 0.2 g of ammonium phosphomolybdate powder was added to it. The optical density of this dye solution was determined with the help of a spectrophotometer (λmax = 615 nm). This beaker was exposed to a 200 watt tungsten lamp. A water filter was used to cut off thermal radiations. 2.0 mL of dye solution was taken out at regular intervals, filtered and its optical density was measured. It was observed that the concentration of janus green B dye decreases with increasing time of exposure. A plot of 2 +log O.D. against time was found to be linear. |
RESULTS AND DISCUSSION
|
Typical Run
|
| It was observed that optical density decreases with increase in time of irradiation and a plot of log O.D. v/s time was a straight line. It indicated that the photocatalytic degradation of janus green B followed a pseudo-first order kinetics. The results of photo catalytic degradation of janus green B are given in Table 1 and graphically represented in Fig. 1. The rate constant of this photocatalytic reaction was determined using the expression |
| k = 2.303×slope |
Effect of pH Variation
|
| The variation of pH on the photocatalytic degradation of janus green B was studied in the pH range 9.5 to 11.5 keeping all other factors identical such as concentratin of dye, amount of semiconductor and light intensity. Under acidic conditions janus green B was not degraded, but in basic range the dye solution was degraded and the colour did not return back even on additin of acid. The value of pH was varied by the addition of H 2SO4 (for acidic range) and NaOH (for basic range) to the dye solution. The results are reported in Table 2. |
| It is evident from above data that the rate of photocatalytic degradation of janus green B increases with increase in pH. The increase in rate of photocatalytic degradation may be due to more availability of the OH- at higher pH values. OH- will generate more .OH by combining with the hole and these hydroxyl radicals are responsible for this photocatalytic degradation. As the pH of the solution was increased, more OH- will be available and these will be adsorbed on the surface of the semiconductor making it negatively charged and as a consequence of repulsive force between two negatively charged species (OH- and electron rich dye). The approach of electron rich janus green B to the semiconductor surface will be retarded. This will result in to a decrease in the rate of photocatalytic degradation of janus green B. |
Variation of Janus green B Concentration
|
| The variation of janus green B concentration on the rate of photo catalytic degradation was studied by taking the concentration of janus green B from 7.6 x 10–5 M to 9.2 x 10–5 M keeping all other factors constant. The result are reported in Table 3. |
| It has been observed that the rate of photocatalytic degradation increases with an increase in the concentration of the dye upto 8.40 x 10-5 M. It may be due to the fact that as the concentration of the janus green B was increased, more dye molecules were available for excitation and energy transfer and hence, an increase in the rate was observed. The rate of photocatalytic degradation was found to decrease with further increase in the concentration of dye. This may be attributed to the fact that the dye starts acting as a filter for the incident light and it does not permit the desired light intensity to reach the semiconductor surface; thus, decreasing the rate of photocatalytic degradation of janus green B. |
Effect of Amount of Semiconductor
|
| The amount of semiconductor powder may also effect the rate of photocatalytic degradation of janus green B and therefore, the amount of semiconductor was varied from 0.1 g to 0.3 g keeping all the other factors identical. The results are reported in Table 4. |
| As indicated from the data, an increase in the amount of semiconductor also increases the rate of photocatalytic reaction up to a certain amount of semiconductor (saturation point). This can be explained on the basis that with an increase in the amount of semiconductor, the surface area of the semiconductor will increase and hence, the increase in the rate of reaction. But after a certain limiting amount of semiconductor ; if the amount of semiconductor is further increased, then it will not contribute to an increase in the exposed surface area. On the contrary, it will increase only the thickness of the layer of semiconductor powder at the bottom of the reaction vessel and thus, the saturation point is reached. |
Effect of Light Intensity
|
| To observe the effect of light intensity all other factors were kept constant. The effect of light intensity on the photocatalytic degradation of janus green B was studied by varying it from 41.0 mWcm-2 to 51.0 mWcm-2. A 200 W tungsten lamp was used for 4 hours for irradiation purpose, which was located 20 cm above the surface of the dye solution. The solutions were positioned at circles of different diameters such as 2, 4, 6, 8 and 10 cm from to center of the light source. The intensity was measured by solarimeter (CEL Model SM- 201). The results are reported in Table 5. |
| The rate of photocatalytic degradation increases as the light intensity was increased. It was found that up to light intensity (51.0 mWcm-2); the rate of photo catalytic degradation increases on increasing light intensity. It may be explained on the basis of number of excited molecules. As more intensity of light falls on ammonium phosphomolybdate semiconductor surface, more number of photons will be available for excitation and, therefore, more electron-hole pairs will be generated on the surface of semiconductor, which in turn, may degrade more dye molecules and thus, the rate of degradation was found to increase with increasing the intensity of light. |
MECHANISM
|
| On the basis of above studies, a tentative mechanism has been proposed for the bleaching of dye by ammonium phosphomolybdate semiconductor. |
| 1Dye0 → 1Dye1 (Singlet excited state) (1) |
| 1Dye1 → 3Dye1 (Triplet excited state) (2) |
| SC → e- + h+ or SC+ (3) |
| h+ + OH- (from base) → .OH (4) |
| 3Dye1 + .OH → Products (5) |
| Dye absorbs the light and gets excited to its first singlet state. This gets converted to triplet state through intersystem crossing. On the other hand, the semiconductor gets excited by absorbing light and an electron is excited from its valence band to conduction band leaving behind a hole. This hole abstracts an electron from OH- ions generating .OH free radical. The dye is bleached by this .OH radical. The participation of .OH radical was confirmed by using scavenger (2-propanol), which almost stops the degradation reaction. |
ACKNOWLEDGEMENTS
|
| The authors are thankful to Prof. Suresh C. Ameta, Former Professor, Department of Chemistry, M. L. Sukhadia University, Udaipur (Raj.) for helpful discussions, and valuable suggestions. |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
Figures at a glance
|
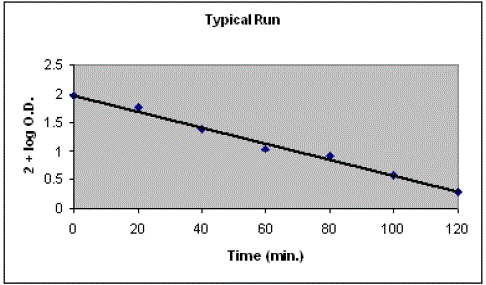 |
| Figure 1 |
|
References
|
- Ameta, R., Vardia, J., Bhatt, C.V. and Suresh Ameta, C. 1997. Sam. J. Chem. 1 : 29.
- Chen, C.C. , Lu, C.S. and Cheng, Y.C. 2006. J. Photochem. Photobiol.181A : 120.
- Chu, W. and Tsui, S.M. 2002. Water Research. 36 : 3350.
- Die vitaleFarbung, L. 1900. eineDarstellungsmethode der Zellgranuls, Arch. mikroskop. Anat. 55 : 558-575.
- Li, F.B., Li, X. Z. and Cheah, K.W. 2005. Environ. Chem. 2 : 130.
- Muneer, M., Das, S., Manital, V.B. and Haridas, A. 1992. J. Photochem. Photobiol. 63A : 107.
- Brown, G.T. and Darwent, J.R. 1984. J. Chem. Soc. Faraday Trans. I. 80 : 1631.
- Mahmoodi, N.M., Arami, M., Limace, N.Y. and Tabrizi, N. S. 2006. J. Collid, Interface Sci. 295 : 159. Mansoori, R.A., Kothari, S. and Ameta, R. 2004. J. Indian. Chem. Soc. 81 : 335.
- Nogueria, R.E.P. and Jardin, W.F. 1993. Solar Energy Mater. Solar Cells. 29 : 109.
- Neppolian,B., Sakthivel, S., Arabindoo, B., Palanichamy, M., Mugugesan, V., Janieson, M. and Serpone, N. 1999. Bull. Cat. Soc. Indian. 9 : 164.
- Sharma, A., Ameta, R., Mathur, R.P. and Suresh, Ameta, C. 1995. Hung. J. Ind. Chem. 23 : 31.
- Sivakumar, T., Shanthi, K., Sankarguru, S.P., Srividya, B., Kiruthiga, P.S. and Rahunathan, R. 1999. Asian J. Microbiol. Bitech. Environ. Sci. 3.
- Silva, M.K., Marques, R.G., Machado, N.R.C.F. and Santos, 1. O.A.A. 2002. Braz. J. Chem. Eng. 19.
|