Keywords
|
| Textile dying Effluents, Adsorption, Malachite Green |
INTRODUCTION
|
| Dyes used in textile industries are highly toxic and carcinogenic in nature . Hence their presence in waste water is unwarranted and it is desirable to remove coloring material from effluents, before their discharge to the environment. Several physicochemical processes like filtration , flocculation, biological stabilization and adsorption by activated charcoal have been developed . Some of these methods are selective , expensive and may need special infrastructure. Among all these treatment processes adsorption is found to be highly effective. Activated Carbon is a highly porous material with large surface area and intrinsic adsorption properties, but it is non regenerable. Thus effective utilization of fly ash as a cheap alternative adsorbent of textile dye and the investigation of its adsorption characteristics is a challenging problem till today . |
| Removal of some selected azo and anthraquinone dyes by adsorption on mixtures of sandy clay loam (SCL) with fly ash and organic matter has been done by Albanis et al. in 2000 . They also achieved 99% adsorption of direct yellow 28. They also reported 98% color removal from various dye using bacterial strain. Colour removal from textile effluent using hard wood and saw dust as adsorbent was tried by Asfour et al. 1985. The potentiality of fly ash as a catalyst in destructive decoluorisation of various dye using Hydrogen Peroxide has been studied by Gupta et al. 1987. Karunanithi et al. 2000 studied the effect of time on color removal using fly ash. They reported 90-99 % removal of Grey and Onion-red color from effluents. In another study3 they reported that the charcoal obtained from coconut shell is not so effective for dye removal. Adsorption characteristics of fly ash for removal of Prusian Brill Blue-M-B was also studied by Srikanth et al. 2000 . Efficiency of fly ash for colour removal from aquous solution of methylene blue and its kinetic behavior was studied by Subanandam et al. 2003. They indicate that adsorption takes place by micelle formation. The present work throws some light on adsorption characteristics of fly ash for the treatment of textile dye containing Malachite- Green . The potentiality of fly ash as an adsorbent has been studied at various experimental conditions. |
Materials
|
| Fly ash obtained from Kolaghat Thermal Power Station is used as the absorbent. The samples were collected from ESP-3 which contains less free Sio2 components as per analysis. Fly ash is a finely divided residue resulting from the combustion of powdered coal or lignite, it is generally acidic in nature. It has a specific surface area which varies from 2500 to 7000 sq. cm/g, particle size range is 120 to 960 microns to less than 5 microns. Specific gravity is found to vary from 2.3 to 2.5 and bulk density 600 to 900 kg/m3 (Srikanth et al. 2000). Malachite green from Qualigens fine chemicals are used as the dye. |
Experimentals
|
| Fly ash has been washed with boiling distilled water, filtered and dried at about, 600ºC and finally sieved to get 63 mesh size. The mean diameter of the particle was found about 0.0057223 mm. |
| Experiments were carried out by shaking various quantities of adsorbents (1 to 10 gms of fly ash) and using different concentrations of effluent from 5 ppm to 20 ppm at various temperature. All the samples were kept in a constant temperature shaker which was running at 100 r.p.m. for different contact time of 15 min, 30 min, 45 min and 60 min. The percentage of adsorption is determined colorimetrically. |
Results and Discussion
|
| The effect of adsorbent amount and the time of contact on the percentage removal of Malachite Green from the effluents of various concentration has been studied and reported elsewhere7. It shows that percentage removal of dye increases with increase of fly ash amount and also with increase of contact time, but after a certain time (about 45 minutes) it remains constant. |
| The effect of concentration on removal of dye was also studied and it was noted that the maximum percentage removal of dye using fly ash is about 88% , which is dependent on dye concentration. This is because at lower concentration, the ratio of available surface area to moles of dye is higher. So adsorption should also be higher as expected for larger amount of adsorbent. But with the increase of solution concentration this ratio decreases due to decreasing adsorption rate. Similar adsorption characteristics have been reported by Srikanth et al. in 2000, and Subanandam et al. in 2003. |
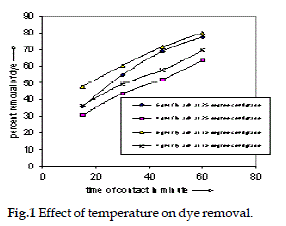
| Effect of temperature on adsorption has been shown in the Fig. 1. Here adsorption studies were done for 20 ppm concentrated solution using two different amount of fly ash ( 4 gm, and 6 gm) at temperature of 15°C and 25°C. For both the cases it was observed that the adsorption rate increases at 15°C ( at the lower temperature), which also indicates that the adsorption is exothermic in nature. |
Adsorption process
|
| The adsorption of malachite Green dye “A” from solution on fly ash “B” may be considered as reversible reaction |
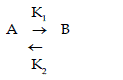 |
| Considering CA0 as the initial concentration of dye in the solution, in moles / litre , CA as concentration of dye in the solution at time “ t”, in moles /litre , and CB is the concentration of dye on the adsorbent, fly ash at time “ t”, in moles /litre. If the equilibrium concentration of solute in the solution and on adsorbent are indicated by CAeq and CBeq, then the rate equation will be as |
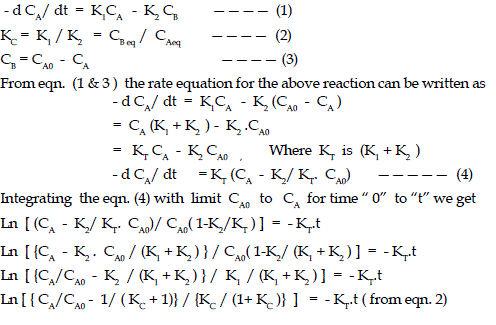 |
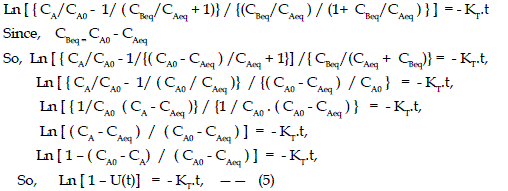 |
| Where U (t) is the fractional attainment of equilibrium and is given by (CA0 - CA)/(CA0 - CAeq), KT is the sum of K1 and K2, where K1 and K2 are the rate constant for adsorption and desorption respectively, KC the equilibrium constant,. CBeq and CAeq are equilibrium concentrations of dye on adsorbent and in solution respectively in moles/L. |
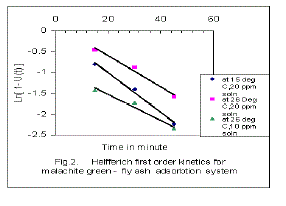
| Using equation (5), ln[1-U(t)] Vs. time “ t ” were plotted at various temperature and concentrations, The linear plot in Fig.2 indicates that the process involves Helfferich first order kinetics. The slope of the graph give the values of KT tabulated in Table 1. Rate constants K1, K2, and KC can be calculated from the equations 2 to 5 , It has been noted from the results that values of rate constants depend both on temperature and concentration of the solution. Rate constant K1, decreases with increase of temperature but increases with decrease of solution concentration, whereas K2 increases with increase of temperature but decreases with decrease of solution concentration. The negative adsorption tendency of the fly ash with increase of temperature also indicates the exothermic nature of this adsorption process. |
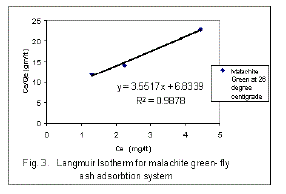
| The experimental results obtained from flyash - malachite green adsorption process at 26°C have been correlated with the following rearranged Langmuir model of adsorption. |
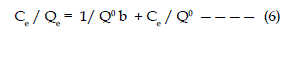 |
| Where Ce is the equilibrium concentrations (in mg/lit.) of dye, and Qe is the amount of dye adsorbed per gram of adsorbent at equilibrium (mg/ gram), Q° and b are Langmuir constants related to the capacity and energy of adsorption respectively. |
| The plot of Ce/Qe Vs. Ce is shown in fig. 3. The linear plot suggest the applicability of the Langmuir Isotherm for this system. It also indicates the formation of monolayer coverage at the outer surface of the adsorbent. The values of Q° and b , Langmuir constants are calculated from the slope and intercepts of the graph and reported in Table 2. |
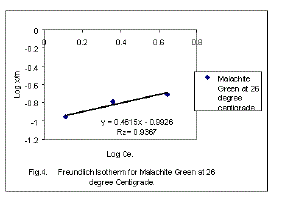
| Applicability of the Freundlich Isotherm for this present system has also been found out by correlating the results using the following Freundlich equation. |
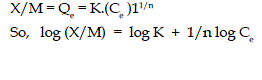 |
| Here X/M or Qe is surface load in (mg/gram). The linear plot of log (X/M) Vs log Ce in Fig 4. also suggests that the system also follows Freundlich Isotherm, The values of n and k , the Freundlich constants are shown in Table 2. |
| The values of correlation coefficient (R2) obtained for Langmuir and Freundlich isotherms are 0.9878 and 0.9367 which indicates that Langmuir’s model is highly applicable than that of Freundlich’s model for the present system. |
Conclusions
|
| 1. Although the efficiency of the activated charcoal is higher than that of fly ash in terms of amount of adsorbent required, yet it has a good potentiality as cheaper adsorbents for dye removal from effluents. |
| 2. Since availability of fly-ash is not restricted, regeneration of used up adsorbents is not necessary. |
| 3. The results obtained from adsorption studies of Mlachite Green by fly ash at various temperature have been correlated with Freundlich Isotherm and rearranged Langmuir model of adsorption isotherm. The linear plot suggest the applicability of both the Freundlich’s as well as Langmuir’s model for this isotherm but Langmuir’s model is more applicable than that of Freundlich’s model for this system . |
Nomenclature
|
| CA0 = Iinitial concentration of dye in the solution, in moles /L |
| CA = concentration of dye in solution at time t , in moles /L |
| CB = concentration of dye on the adsorbent, at time “ t” , in moles /L |
| CAeq = equilibrium concentration of dye in the solution, in moles /L |
| CBeq = equilibrium concentration of dye on adsorbent , in moles /L |
| U (t) = fractional attainment of equilibrium |
| K1, K2 = the rate constant for adsorption and desorption |
| KC= (K1/K2) = equilibrium constant, |
| X/M = Qe = is surface load, in mg/g. |
| Ce= equilibrium concentrations of dye, in mg/L |
| Qe = amount of dye adsorbed per gram of adsorbent at equilibrium, in mg/g, |
| Q°, b = are Langmuir constants |
| n , k = Freundlich Isotherm constant, R = correlation coefficient. |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |
References
|
- Albanis, T.A. Hela, D.G., Sakellarides, T.M. and Danis, T.G. 2000. Removal of dyes from aqueous solutions by adsorption of mixtures of fly ash and soil in batch and column techniques. Global Nest : Int. J. 2 (3) : 237- 244.
- Asfour, H.M., Nasser, M.M., Fadi, O.A. & E.I. Geundi, M.S.1985. Colour removal from textile effluent using hard wood and saw dust as adsorbents. J.Chem. Tech. Biotechnol. 35A : 28-35.
- Das, R. 2002. Treatment of textile dyeing industry effluents using different adsorbents. Proc. Indian Chemical Engineering Congress.
- Gupta, G.S., Prasad, G., Pandey K.K. and Sing, V.N. 1987. Water,Air,- Soil Pollut. 37 :13. Karunanithi,T., Bhoopathy, S., Palaniappan, M., Karuppaiya, S. and ArunPrakash,T.K 2000. Treatment of textile dyeing industry effluent using different adsorbents. Proc. Indian Chemical Engineering Congress.
- Karunanithi, T., Ganesh, T.S., Venkatachalem, L. and Arun Prakash, T.K. 2000. Treatment of textile dyeing industry effluent using fly ash. Proc. Indian Chemical Engineering Congress.
- Srikanth, A.V., Reddy, Ramana, Sailu, S.V. and Basava Rao, V.V. 2000. Treatment of textile dye effluents by fly ash adsorption studies. Proc. Indian Chemical Engineering Congress.
- Subanandam,K. &VelagtyudhamMuthu, R. 2003. Kinetics studies on adsorption of methylene blue on lignite fly ash. Jr. Industrial Pollution Control. 19 (2).
|