Keywords
|
| Poly chlorinated biphenyl, Nano clay, Adsorption. |
INTRODUCTION
|
| The occurrence of polychlorinated biphenyl (PCBs) in soil and water systems is currently a major problem of the global concern because of their harmful impact on ecosystem health and on the safety of human food supplies (Sawicki, 2006). Moreover, PCBs are the typical persistent organic toxins present in the natural environment ubiquitously, and they are high toxic and resist to degradation and have high bioaccumulation property. Therefore, the remediation of these contaminants is essential to promote, public health, environmental quality, and the economy (Bedard, 2003). Traditional remediation for these contaminants has serious limitations and high costs. Several remedial technologies have been used to destroy, and remove PCBs from contaminated soil and water, Such as incineration, thermal desorption, Chemical dehalogenation, solvent extraction, soil washing, chemical oxidation, vitrification, bioremediation (Boehm, 2006). |
| Additionally, the numerous applications of clay minerals in soils and the formation and protection of the environment are in general key issues of studies during couple of few decades (Kuhnel, 1990). Clay re hydrous alumina silicates broadly defined as those minerals that make up the colloid fraction (<2μm) of soils, sediments, rocks and water. The high specific surface area, chemical and mechanical stability, variety of structural and surface properties, higher value of cation exchange capacities, etc., make the clays and nano clays an excellent group of adsorbents. Application of nano clay showed numerous in the different areas of the environmental sciences particularly in the removal of variety of organic and inorganic pollutants (Bhattacharya 2006). The aim of this study is to evaluate the use of montmorillonite nano clay, in contaminated soil to adsorb PCBs contaminant. |
Materials and Methods
|
Material
|
| All the solvents were of GC grade (obtained from Merck) and were used without further purification. Deionized water was obtained from the Millipor ultra pure water system. Decon 90® solution purchased from Decon Laboratories Ltd. was used to wash all glassware. |
Properties of PCBs
|
| Poly chlorinated biphenyl was purchased from Sigma Adlrich. The PCBs that used in this study is 2-chlorobiphenyl. Its purity is 99.2%. Chemical properties of PCB is presented in Table 1. |
Properties of Nano Clay
|
| The material properties of nano clay used in this study is described in Table1. The nano clay is Cloisite® Na+, purchased from Nano clay was purchased SOUTHERN CLAY PRODUCTS. Nano clay is Cloisite® Na+, that is a natural montmorillonite. Some properties of Nano clay are shown in Table 2. |
Soil properties
|
| The residual soil used in this study is obtained from soil located in University Kebangsaan Malaysia, Bangi, Selangor. The soil was taken from about 200 mm - 400mm below the surface. In the laboratory, it was air dried for 15 days, roots separated from the bulk soil and it was stored in polythene bags until ready for use. Some soil properties are shown in Table 3. |
Batch experiment
|
| Adsorption tests were also conducted using a simple but effective batch adsorption method. Experiments were conducted to compare the efficacy of concentration of Nano clay to adsorb PCBs. PCBs solution was prepared by mixing PCBs in n-Hexane (because of very low water solubility of PCBs. The concentration of solution was 10, 20, 30, 40, 50, 60 ppm. The concentration of nano clay was added to PCBs solution was 0.03, 0.06, 0.09, 0.12, 0.24 and 1.2 gram. Batch adsorption test was carried out in glass vials with 60 mL solution (at room temperature). Solution mixed with Nano clay was shaken (150 rpm) for 22 hours. Solution samples were filtered and transferred to 1.5 mL Gas Chromatography bottles for gas chromatography analysis. PCBs concentrations were measured on gas chromatography (ECD). These operation conditions were chosen after optimization of each parameter and samples were tested in triplicate. The amount of PCBs adsorbed (Q) was then calculated using the following equation: |
 |
| Where Ci is the initial concentration, Ce is the final or equilibrium concentration, V is volume of solution, and m is the mass of soil (12g). The experimental data were fitted to two commonly used adsorption isotherm equations namely the Freundlich and Langmuir models. These two models are widely used, the former being purely empirical and latter assuming that maximum adsorption occurs when the surface is covered by adsorbate. The Freundlich and Langmuir equation commonly presented as equations 2 and 3. |
 |
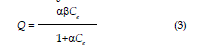 |
| where Q is the amount of solution adsorbed on the adsorbent (mg/kg), Ce is the concentration at equilibrium (mg/L), a and ? are the Langmuir constants, and n and k are Freundlich constants, these models are arranged to linear form as follows: |
 |
 |
| All the experiments were carried out in completely randomized block designs with three replications. All data were statistically analysed with single factor analysis of variance (ANOVA). Any significance reported represents P = 0.05. |
RESULTS AND DISCUSSION
|
| The results of the adsorption test conducted in this study are shown in Figure 1. Figure 1 shows linear adsorption isotherm for different concentration of PCBs in contact with various amount of nano clay. Figure 1 illustrates that adsorption of PCBs increase when amount of nano clay is increased. The maximum adsorptions were observed at the high levels of nano clay because of the maximum capacity of the adsorption sites. As shown in Figure 1, the maximum percentage of adsorption for the parameters in this study is 77%. |
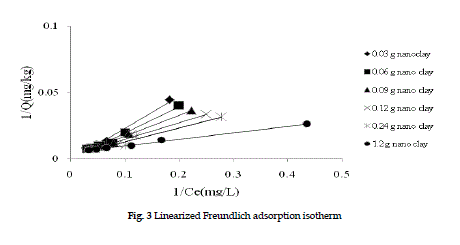
| The linearized adsorption isotherms (Fruendlich and Langmuir) of PCBs on nano clay are shown in Figures 2 and 3. |
| Figure 2 shows the Freundlich isotherm graphs for all data. At 1.2 g of nano clay, the Kd is 7.68 kg/L and correlation coefficient, R,2 is 96%, indicating a good correlation coefficients. Figure 2 shows the experimental data are well simulated in Freundlich model. The Q (adsorption amount of PCBs) increased by increasing the equilibrium concentration. This phenomenon could be explained by the increased surface are of the nanomaterials which causes more adsorption. The more nano clay that were present, the more available surface area and more PCBs adsorption were observed. Figure 3 indicates the experimental data in Langmuir model. The a and β values are 1.07 L/mg and 208.3 mg/kg at 1.2g of nano clay. Therefore the maximum of solute that can be adsorbed by nano clay is 2083.mg/kg. The correlation coefficient for Langmuir adsorption isotherm R2 is 98%. |
| In this study, the adsorption of PCBs to nano clay (Cloisite® Na+) was investigated. Batch adsorption test was conducted at 10 to 60 mg/L of concentration of PCBs and various amount of nano clay. The gas chromatography system was used for analysing the result of experiments. The adsorption of PCBs onto nano clay was examined by employing the Freundlich and Langmuir isotherms. The maximum percentage of adsorption of PCBs by nano clay was 77%. The present investigation shows that montmorillonite nano clay (Cloisite® Na+), can be employed as effective adsorbent for removal of PCBs from soil and water. The removal of PCBs followed the Freundlich and Langmuir, and was found the initial amount of nano clay has important effect on adsorption procedure. |
ACKNOWLEDGEMENT`
|
| This research was financially supported by Project No.UKM-KK-02-FRGS0002-2007. The authors gratefully acknowledge this financial support. |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |
References
|
- Sawicki, R. and Mercier, L. 2006. Evaluation of mesoporouscyclodextrin-silica nano composite for the removal of pesticides from aqueous media. Environ Sci. Technol.15.40 (6) : 1978-1983.
- Bedard, D.L. ...............? Polychlorinated biphenyls in aquatic sediments:environmental fate and outlook for biological treatments. In: Dehalogenation: MicrobialProcesses and Environmental Applications, M.M. Häggblom and I.D. Bossert, eds, Kluwer Academic Publishers, Boston, pp. 443–465.
- Boehm, F. 2006. Nanotechnology in Environmental Applications, Report NAN039A, BCC Research, Norwalk.
- Bhattacharya, K.G. and Gupta, S.S. 2006. Adsorption of few heavy metals and modified kaolinite and montmorillonite. Separation and Purification Technology. 50 : 388-397.
- Freundlich, H. 1926. Colloid and Capillary Chemistry. London, Methuen and co., Ltd
- Kuhnel, R. A. 1990. The modern days of clays. Applied Clay Science. 5 : 135-143.
- Langmuir, I. 1918. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 40: 1361-1382.
|