Keywords
|
| Temperature, pH, Salinity, Alkalinity, Dissolved Oxygen and Estuary |
INTRODUCTION
|
| The physico-chemical factors contribute the making of the particular ecosystem which determines the trophic dynamics of the water body. Estuaries are physico chemically unique as they show strong variations in salinity, temperature, pH, dissolved oxygen, redox potential and the amount and composition of particles (Chapman and Wang Feiyue, 2001).They represent the outfall regions of river and represent transitions between fluvial and marine environment, where tidal actions influence the mixing of freshwater and saltwater. The rate of mixing depends upon channel geometry, the relative amount of freshwater and tidal inflow, wind and the difference in densities of two types of waters (Dutt, 1987). The physical parameters assess the changes that may take place in well-defined characteristics as a result of the addition of potential pollutants. |
| Among the various abiotic factors, the factors such as salinity, temperature and pH are considered as most important since the variations of which would definitely pose a threat to biota (Menon et al. 2000). The fate and effect of different pollutants entering into the estuaries can be obtained from the existing relationship with salinity and other physico-chemical parameters. So it is worthwhile to acquire the hydrographical features of the estuaries at different seasons as it determines the tropic dynamics of the water body. Thus the present study focuses the physico-chemical features of a ramsar lake “Veli” in the Kerala coast |
MATERIALS AND METHODS
|
Collection of Samples
|
| Water samples for a period of one year from April 2000 to March 2001 were collected. Sampling was done during morning hours between 6 AM and 10 AM. Surface water samples were collected using a clean plastic bucket and the bottom water with a van-dorn bottom water sampler at a depth of one meter from the surface. The collected water samples were transferred to the pre-cleaned, polythene bottles and stored in the refrigerator. The water for the estimation of dissolved oxygen was fixed in the field itself and the samples used for the analysis were filtered by Millipore filtering unit through Whatman GF/C filter paper of 0.5m porosity. |
| Temperature of surface and bottom water was recorded in the site itself using a high quality Celsius thermometer of ± 0.5°C accuracy. The hydrogen ion concentration (pH) of water sample was measured in the laboratory using Elico-model L1 - 10 pH meter having ± 0. 01accuracy. The Mohr-Knudsen titration method using potassium chromate indicator was followed to measure the salinity (Grasshoff, 1981). The alkalinity of the sample was estimated by back titration method by Trivedy and Goel (1986). Dissolved oxygen was determined by classic Winkler’s titration method (Grasshoff, 1981). |
RESULTS
|
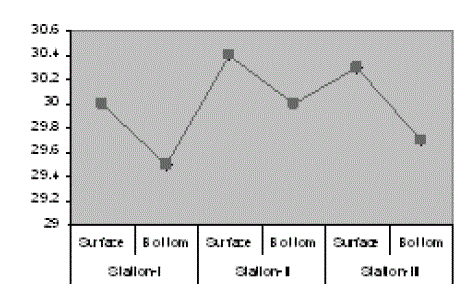
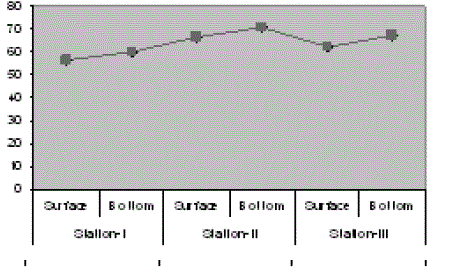
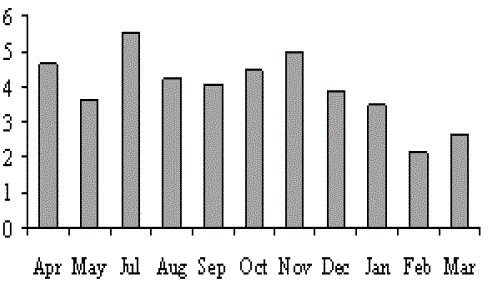
| The results of the water quality parameters such as temperature, pH, salinity, alkalinity, dissolved oxygen, were interpreted in Figs. 1-10. The mean temperature ranged between 29.5 °C in the bottom of station I and 30.4 °C in the surface of station II with the mean value of 29.98 °C. The mean pH was 7.64 which ranged between 7.56 in surface of station III and 7.7 in the surface of station I. The mean salinity of Veli was 5.95 ppt which ranged from 5.28 ppt in surface of station II to 6.71 ppt in the bottom of station III. In Veli, the mean alkalinity was 63.89 mg/L which ranged between 56. 17 mg/L in the surface of station I and the bottom of station II with 71 mg/L. The DO in Veli ranged between 5.66 mg/L in bottom of station I and 6.8 mg/L in the surface of station III with the mean value of 6.35 mg/L. |
DISCUSSION
|
| The fall in water temperature in monsoon may be due to the fewer incidences of radiation, heavy freshwater discharge and precipitation. The rise in temperature noticed in premonsoon was due to the influence of hot climatic conditions and concentration of more pollutants. Similar situations of high temperature during premonsoon and low temperature during monsoon months have been reported by Nair et al. (1984).Temperature variations noticed between the stations may possibly be due to the state of pollutants and the geomorphology of the estuary. The pH varies according to the level of mixing of sea water with the fresh water. The high values at the marine zone were due to the coagulation of colloidal particles which shifts the pH towards the alkaline side (Nair et al., 1983).During post monsoon, comparatively higher pH values were observed due to the mixing of sea water and biological activity (Day, 1981). The mean pH of Veli Lake was towards acidic and this was due to the discharge of acid effluents from the Travancore titanium industries (Nair et al. 1984). The oscillation of pH towards the acidic phase or near to neutrality at all stations during the monsoon could be attributed to heavy river discharge and land run off. |
| Salinity, the most fluctuating parameter in the estuary depends on the topography, state of tide, time of the year, intensity of rainfall and the extent of fresh water flow. Geetha Bhadran (1997) reported that salinity of an estuary is being controlled diurnally by the entry of sea and freshwater from both sides and variations in salinity may be controlled by the nature of connection of the estuary. Surface salinity within the estuary was observed to be lower than that of the bottom in most of the months due to the density of ions that sink to the bottom leads to salinity stratification. Wellershaus (1971) reported that the freshwater could no longer fill up the whole basin of the backwater from surface to the bottom and it was only limited to the surface layer. The near bottom counter current of the sea water leads to the intrusion of sea water in the deeper channels and the salinity increased continuously with the depth. The increased salinity during pre monsoon could be attributed to increased temperature and wind resulting in greater evaporation (Reddy and Hariharan, 1985). The monsoon season with more or less high salinity was due to the fact that the intrusion of sea water controlled the freshwater flow from the link canals and the post monsoon with less salinity gradients. |
| The high values of alkalinity may be attributed to the dissolution of calcium carbonate from the sediments. Like salinity, alkalinity also exhibited high values in bottom and in the bar mouth region. Geetha Bhadran (1997) reported that alkalinity in natural waters was due to the presence of free hydroxyl ions and hydrolysis of salts formed by weak acids and strong bases such as carbonates and bicarbonates. Padma and Periakali (1999) reported that increase in alkalinity during post monsoon period was due to the input of freshwater and dissolution of calcium carbonate and bicarbonate ions in the water column. The higher surface DO levels in all the stations in most of the months suggest that the surface water tends to attain saturation levels of oxygen because of the direct diffusion of atmospheric oxygen and super saturation or depletion of oxygen which may be due to the result of photosynthetic and respiratory activities of marine organisms. The combined effects of winter cooling and high photosynthetic activity lead to the increase of DO during monsoon (Padmavathi and Satyanarayana, 1999). |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |
References
|
- Chapman, P.M. and Wang Feiyue, 2001. Assessing sediment contamination in estuaries. Environ. Toxicol. Chem. 20 (1) : 3-22
- Day, J.H. 1981. Estuarine Ecology. A.A. Balkema (Eds.), Rotter dam, 58pp.
- Dutt, D.K. 1987. Estuarine Environment and ground water pollution. Proc. Natn. Sem. Estuarine Management, Trivandrum, pp.l-8.
- GeethaBhadran, 1997. Heavy metal pollution in Ashtamudy estuarine system. Ph.D. Thesis. Department of Aquatic Biology & Fisheries, University of Kerala, Trivandrum, India, 212pp
- Grasshoff, K., Ehrhardt, M. and Kremling, K. 1983. Methods of Sea Water Analysis. II edition, Verlag. Chemie. 419pp.
- Menon, N.N., Balchand, A.N. and Menon, N. R. 2000. Hydrobiology of the Cochin backwater system – a review. Hydrobiologia. 430 (1-3) : 149-183.
- Nair, N.B., Azis, P.K.A., Arunachalam, M., Kumar, K.K. and Balasubramaniam, N.K. 1984. Ecology of Indian Estuaries. VIII. Inorganic nutrients in the Ashtamudi estuary. Mahasagar. 17 (1) : 19-32.
- Nair, N.B., Kumar, K.K., Arunachalam, M., Azis, P.K.A. and Dharmaraj, K. 1983. Ecology of Indian estuaries: Distribution of organic carbon in the sediments of the Ashtamudi estuary. Indian J. Mar. Sci. 12 : 225-227.
- Padma, S. and Periakali, 1999. Physico-chemical and geochemical studies in Pulicat lake, east coast of India. Indian J. Mar. Sci. 28 : 434-437.
- Padmavathi, D. and Satyanarayana, D. 1999. Distribution of nutrients and major elements in river line, estuarine and adjoining coastal waters of Godavari, Bay of Bengal. Indian J. Mar. Sci. 28 : 345-354.
- Reddy, H.R.V. and Hariharan, V. 1985. Physico-chemical parameters of Netravathi- Gurupur Estuary, Mangalore. Environ. Ecol. 3 (4) : 530-534.
- Trivedy, P.K and Goel, P.K 1986. Chemical and Biological Methods for Water Pollution Studies. Environmental Publications, Karad, 220pp.
- Wellershaus, S. 1971. On the Hydrography of the Cochin Backwater, A South Indian Estuary. J. Mar. Biol. Ass. India. 14 (2) : 487-495.
|