Keywords
|
| Fluoride, Adsorption Isotherm, Impregnations, Kinetic Model, Fly-ash, Lime Slurry |
INTRODUCTION
|
| Endemic flourosis and acute health related problems in India results from high fluoride levels in ground water Problems with the high fluoride concentration in the ground water resources has become an important health related geological issue in the various parts of India (Agrawal et al., 1997), where nearly 3 million people are reported to consume excess fluoride containing water (Maithani et al., 1998). Above the WHO recommended maximum limit 1.5 mg/L (Apambire et al., 1997). In some parts of Rajasthan in India, fluoride concentration reaches up to 18 ppm (Sompura, 1998) causing severe diseases, i.e. skeletal and dental fluorosis (Choubisa, 1998) in both human beings and animals. Health problems are more severe in rural areas where costly defluoridation techniques such ion- exchange (Chaturvedi et al., 1998), reverse osmosis (Joshi et al., 1992), nano- filtration (Simons, 1993), electrodiaysis (Adhikary et al.,1989) and Donnandialysis ( Ruiz et al., 2003) cannot be adopted due to higher cost. In recent years, attention has been paid to develop low cost techniques for removal of fluoride from potable water. Low cost materials like bone charcoal(Kiledar and Bharagava, 1993), cement paste (Kang et al., 2007), brick powder (Yadav et al., 2006), various clays, plaster of Paris (Gopal and Elango, 2007) fly-ash etc. have been studied as adsorption catalysts for deflouridation of fluoride enriched water. |
| Large quantities of solid waste fly-ash is produced during the combustion of coal in Kota Super Thermal Power Plant, Kota Rajasthan. Another solid waste in the form of Kota stone (Lime) slurry / sludge is also produced in huge quantity from the Kota stone industries and dumped directly on open land and pollute the environment. |
| Abundant availability of fly-ash and lime slurry inspired us to develop low cost, effective adsorbent material for removal of fluoride from drinking water, which is mainly ground water in large portion of rural areas in Rajasthan, the worst affected one by chronic fluoride related health diseases. |
| During the present study, a noble adsorbent material is being developed by impregnating lime stone slurry on to F-type fly-ash with silica and Alumina>70%. The synthesized adsorbent material is characterized for its structural, mineralogical, morphological and physic-chemical properties. |
| Batch adsorption studies are carried out systematically in terms of process parameters such as initial concentration, pH and adsorbent dose. Langmuir and Freundlich adsorption isotherms are applied. Various kinetic models are tested to find out the suitable one to represent fluoride removal kinetics over lime stone impregnated fly-ash. |
Materials and Methods
|
| F- type fly-ash was collected from Kota Super Thermal Power Station (KSTPS), Kota. Lime stone slurry waste was procured directly from stone cutting industries at Kota. |
| The adsorption catalyst was synthesized by mixing fly-ash and lime slurry in a fixed ratio of 3:1. The mixture was stirred continuously for 5 hrs maintaining the temperature. The mixture was then filtered and dried at 100 °C for 24 hrs to remove excess water. The dried material was milled at 600 rpm for one hour for uniform sizing and increasing surface area. This material was finally calcined at 550 °C for 6 hours to stabilize the lime content on fly-ash for increased adsorption capacity of fly-ash. |
| The stock solution of 100 mg/L fluoride was prepared by dissolving 221 mg of anhydrous NaF (Merck, India) in 1.0 litre of distilled water. fluoride removal studies were conducted using batch adsorption technique. In which, a fixed amount of adsorbent LSFA, (0.5g) was added to 100 mL of fluoride solution of varying concentration taken in 100 mL stoppered conical flasks, placed in a thermostated agitation (30 °C) assembly. The solution was agitated continuously (600 rpm) at constant temperature. The adsorbate uptake qe (mg/g), was calculated using equ.(1) |
 |
| The Kinetics was followed by with drawing 0.5 dm -3 aliquot samples at different intervals. The adsorbent was separated from aliquot by filtration through whatman 42 no. filter paper. Residual concentration of fluoride ion was estimated using ion meter with fluoride ion selective electrode. Experimental runs were observed with initial rapid adsorption trends. |
| FT-IR spectra of different samples were recorded using a Bruker FT-IR spectrophoto-meter (Tensor-27) with DRS. FT-IR spectra of pure fly-ash and LSFA catalyst were recorded in the range 400-4000 cm-1. |
| X-ray diffraction pattern were obtained by a Phillips X-ray diffrectrometer (Phillips Expert) using CuKα Radiation ( λ =1.54056A°). Samples were scanned at 2 range of 0° to 80° at a scanning rate of 0.04S-1(16). Morphological studies were performed using scanning electron microscope (PHILIPS, XL30 SEM). |
Results and Discussion
|
Physico-Chemical Characterization
|
| The result of physico-Chemical analysis of FA is given in Table 1 |
XRD Analysis
|
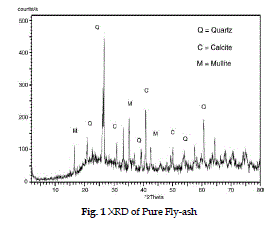
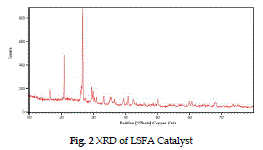
| The XRD pattern of raw fly-ash (fig.1) shows the presence of crystalline phases eg. quartz, mullite, hematite, calcite etc. in the range of 300 - 500. In fig. 2 after the loading of lime slurry on fly-ash in LSFA catalyst, the amorphous silica of fly-ash reacts with the lime slurry to form some new peaks at 310, 320, 370, 470 for the calcium hydrates phase and at 360, 450 representing magnesium hydrate phase (Roxana et al. 2000) by comparing the Fig. 1 and 2, it is clear that crystallinity of the fly-ash is increased on impregnating with lime stone slurry due to presence of crystalline Ca and Mg silicate phases. |
FT-IR analysis
|
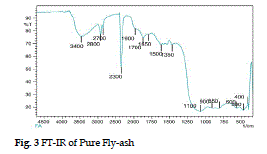
| The FT-IR spectrum on fly-ash in Fig. 1 shows a broad band between the 3400 and 3000 cm-1 which indicate the presence of surface–OH groups (–Si-OH) and adsorbed water molecule on the surface. The spectra also show a broad range of bands from 1055 cm-1 to 1100 cm-1, which is attributed to modes of Si-O-Si asymmetric band stretching vibration. |
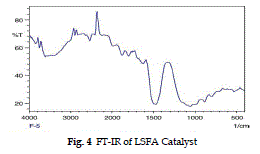
| The FT-IR spectrum of LSFA catalyst in Fig. 2 shows a peak at 1650 cm-1 , due to bending mode of water molecule. The adsorption peak at around 999 cm-1 shows the presence of Si-O-Ca bond (Majumdar and Raki, 2006), and a broad instance band at 3600- 3200 cm-1 indicates assumed that the Si-O-Mg phase is formed in the catalyst. . At high temperature up to 250 0 C the molecular water is removed while structural – OH ions remain associated in the calcium and SiO2 skeleton up to 700 °C (Richar elson, 2008). |
SEM analysis
|
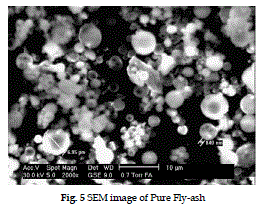
| The SEM pictures of raw and lime slurry impregnated fly-ash are shown in fig. 5 and 6 respectively. Raw fly-ash shows the presence of hollow ceno-spheres, irregularly shapes, unburnt carbon, mineral aggregates and agglomerated particles. The SEM image of lime slurry impregnated fly-ash catalyst (Fig. 6) show dense particles with distribution of varying particles size deposited lime slurry, with clearly visible on the external surface of fly-ash particles. |
Kinetic Modeling
|
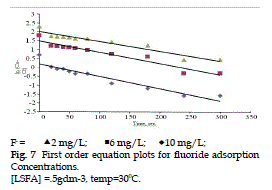
| The adsorption data were fitted at five different Kinetic models using least square regression analysis. The values of slope, correlation coefficient (R2) , and standard errors of estimate (SEE) are given in Table 3. On the basis of high R2 and low SEE, the most favourable model for fluoride adsorption kinetics is the first order (Fig. 7), while two-rate constant and elovich equation are completely rejected. |
Adsorption Isotherms
|
| Adsorption Isotherm helps in determining the feasibility of catalyst as an adsorbent for remediating fluoride ion in water. |
| The adsorption data for removal of F - at [LSFA] 0.5 gm dm-3 at 300C have been tested for Langmuir and Freundlich adsorption isotherms. |
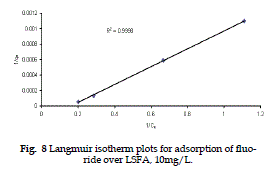
| Langmuir isotherm is valid for single monolayer adsorption, based on the assumption that all adsorption sites have equal affinity for molecules of the adsorbate in the plane of surface. |
| The linear form of the Langmuir isotherm can be expressed as, |
 |
| If the plots, 1/qe V/s 1/Ce are linear the essential characteristics Langmuir isotherm can be expressed in terms of a dimensionless separation factor (r) (Kanan and Meenakshisundaram, 2002), which describes the type of isotherm and is defined by 1/(1+b Co) (Webar and Chakravarti, 1974). |
| The different types of isotherms can be described on the basis of r values (r=>1 unfavourable, r = 1 linear, r < 0, r < 1 is favourable and r=0 irreversible) |
| It is clear from Table 2 that the r values for the present experimental plots are in the range of 0.19 to 0.31, which favours the adsorption of sodium fluoride on LSFA. |
 |
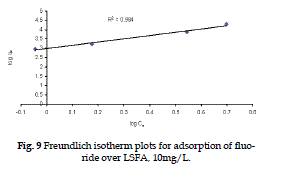
| Figure 9 represents the linear Freundlich adsorption isotherm plotted using equation (4). |
 |
| The plot of Freundlich model of adsorption yields a straight line with slope 1/n and intercept log Kf. The values of Langmuir and Freundlich constants are given in Table (2), a smaller value of 1/n, points out a better adsorption mechanism and formation of relatively stronger bond between and LSFA (Mehrotra et al., 1999) |
Conclusion
|
| The results of the study conclude that fly-ash impregnated with Kota Stone lime slurry waste can be used for the effective removal of inorganic fluoride from the drinking water. As fly-ash and stone slurry both are waste and bear either low or no cost, the adsorption catalyst synthesized (LSFA) using both these materials is cost effective and can replace other costly adsorbents used commercially. |
| The kinetic results are best fitted on first order kinetic model and regression analysis of the equilibrium data fitted the Langmuir and Freundlich isotherms. |
Acknowledgement
|
| XRD and SEM analysis was carried out at sophisticated analytical Instrumentation facility Centre, Punjab University Chandigarh, India. |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
 |
 |
 |
 |
| Figure 6 |
Figure 7 |
Figure 8 |
Figure 9 |
|
| |
References
|
- Adhikary, S.K., Tipnis, U.K., Harkare, W.P. and Govinddan, K.P. 1989 Defluoridation during desalination of brakish water by elestrodialysis. De salination. 71: 301-312.
- Agrawal, V. Vaish, A.K. and Vaish, P. 1997 Ground waterquality:focus on fluoride and fluorosis in Rajasthan.Current Science. 73 (9) : 743-746.
- Apambire, W.B., Boyle. D.R. and Michael, F.A. 1997 Geochemistry,genesis and health implications of fluoridein ground water from western part of Sirohi district,Rajasthan and its crippling effect on human health.Current Science. 74 (9) : 773 - 777.
- Choubisa, S.L. 1999. Some observations on endemic fluoroalein domestic animals in southern Rajasthan (India).Int. J Environ Stud. 56 : 703-716.
- Chaturvedi, A.K., Pathak, B.N. and Singh, Y.N. 1988 Fluorideremoval from water by adsorption on Chinaclay. Appl.Clay Sci. 3 : 337-346.
- Gopal, V. and Elango, K.P. 2007 Equilibirum, kinetic andthermodynamic studies of adsorption of fluoride onto plaster of paris. J. Hazard, Mat. 141 : 98-105.
- Joshi. S.V., Mehta, S.H. and Rao, A.P. 1992 Estimation ofSodium Fluoride using HPLC in reverse Osmosisexperiments. Water Treat. 7 : 207-211.
- Kang, W.H., Kim, E.I., Park, J.Y. 2007 Fluoride removalcapacity of cement paste. Desalination. 202 : 18-44.
- Kiledar, D.J. and Bharagava, D.S. 1993 Effect of stirring rateand temperature on fluoride removal by fishbonecharcoal. Indian J. Environ. Health. 35 : 81-87.
- Kanan, N. and Kshisundaram, M. 2002 Adsorption of CongoRed on various activated carbons –A comparativestudy. Water Air Soil Poll. 138 : 289-305
- Mehrotra, R. Kapoor, B.S. and Narayan, B., 1999 Defluoridationof drinking water using low cost adsorbent.Indian J. Environ. Health. 41 (1) : 53-58.
- Majumdar, S.C. and Raki L. 2006. Preparation thermalspectral and micorscopic studies of calcium-silicatehydrate-poly(acrylic acid). Nanocomposite materials.J Therm. Anal. Cal. 85 (1) : 99-105.
- Maithani, P.B., Ravindra, G, Banerjee, R., Balaji, B.K., Ramchandran,S. and Singh, R. 1998. Anomalous fluoridein ground water from western part of Sironi district,Rajasthan and its cripping effects on human health.Current Science. 74 (9) : 773-777.
- Simons, R. 1993. Trace element removal from ash damwater by nanofilteration and diffusion dialysis. Desalination. 89 : 325-341.
- Qureshi, S.Z., Khan, M.A. and Rahman, N. 1995 Removalof fluoride ion by zirconium (IV) or seniate vanadateusing ion selective electrode. Water Treat. 10 : 307-312.
- Ruiz, T. Pessin, E., Hichour, M. and Sandeaus, J. 2003 Modelizationof fluoride removal in Donmam dialysis. J.Member. Sci. 212 : 113-121.
- Sompura, K. 1998. Study on Prevalence and severty ofchronic fluoride intoxication in relation to certaindeterminants of fluorosis [dissertation], Udaipur(Rajasthan); M.L. SukhadiaUniversity.
- Richarelson, I.G. 2008. The calcium silicate hydrates. Cem.Con Cr. Res. 38 : 137-158.
- Weber, T.W. and Chakravarti, R.K. 1974 Pore and solidDiffusion Modes for fixed Bed adsorber. J.Am. Ins.Chem. Eng. 2 : 228-238.
- Yadav, A.K. Kaushik, C.P., Haritash, A.K., Kansal, A.and Rani, N. 2006. Defluoridation of groundwaterusing brick power as on adsorbent. J.Hazard Mater.128 : 289-293.
|