Keywords
|
| Corrosion, Inhibition, Polarization, Mild steel, Saraca Indica leaf |
INTRODUCTION
|
| Corrosion of metals is a serious problem in water distribution systems. Corrosion increases the operational difficulties, maintenance cost and reduces the service life of the system. The corrosion product in water will affect the quality of water and also result in increased pipeline chocking (Buchweishaija and Mhinzi, 2008; Aiman and Ehab, 2007; Shams, 1986). Corrosion leads to pipeline leakages which require plugging or replacement of corroded pipes. There are many techniques used to prevent corrosion of mild steel in aqueous environment and most common technique is the use of corrosion inhibitors. Organic compounds, especially those having N, S and O showed significant inhibition efficiency. The organic compounds adsorbed on the metal surface and there by forming a protective layer which prevents further oxidation of the metal (Sharma et al. 2009; Abd El-Rehim et al. 1999). Unfortunately most of these compounds are not only expensive but also toxic to living beings (Raja and Sethuraman, 2008). Plant extracts have become important as an environmentally acceptable, biodegradable, readily available and renewable source for wide range of inhibitors (Saratha et al. 2009). The corrosion inhibition effect of many naturally occurring substances had been reported in literature (Broo et al. 1999; Orubite et al. 2004; Olusegun et al. 2009; Chauhan et al. 2007; Saratha and Meenakshi. 2010; Smita and Mehta, 1999; Beenakumari, 2010, 2011). The present research work focused to develop a safe and cheap corrosion inhibitor for drinking water systems. The inhibitor selected for the present study was the extract of the asoka leaf, which is environmental friendly, and also has some good medicinal value (Pradha et al. 2009). In many cases, the presence of E. Coli bacteria is reported in drinking water. The asoka leaf extract can act against E. Coli bacteria present in water (Sarojini et al. 2011; Seetharam et al. 2003). Hence addition of asoka leaf extract on drinking water distribution system not only inhibit the corrosion of pipe line but also improve the quality of drinking water. The corrosion rate was measured at different intervals of immersion of mild steel sample in electrolyte to understand the mechanism of inhibition. |
Experimental Methods
|
Material
|
| The specimens (2 x 1 x 0.2 cm) were produced from strips of mild steel. The composition of the metal is given in Table 1. The specimen were polished by using different metalo-graphic grade of emery papers, ultrasonically degreased by using isopropanol, cleaned by water, dried and store in desiccators. |
The medium selected for study
|
| The medium selected for the study was ordinary tap water used for drinking purpose. The characteristics of water are given in Table 2. The calcium and magnesium present in the water sample were analyzed by EDTA titration. The iron present in the water was analyzed by Atomic Absorption techniques using Atomic Absorption Spectrophotometer of Perkin Elmer (AA200). The anions like, chloride ion present in the medium was analyzed by titration with silver nitrate and sulphate by turbidimetric method using Thoshniwall turbidimeter. |
The Inhibitor selected
|
| The corrosion inhibitor used was the extract of the asoka leaf. The asoka leaf contain tannin, flavanoids, carbohydrates, glycosides, proteins, steroids, alkanoids and amino acids (Saha et al. 2012). The concentration of the inhibitor varies from 0 to 1000 ppm (v/v). For each experiment fresh water solutions as well as freshly polished metallic samples were used. The experiment was duplicated to get the concordant results. |
Electrochemical measurements
|
| Cathodic and anodic polarization curves were recorded on the metal electrode surface (1cm2) immersed in aerated drinking water by sweeping at a rate of 1 mV/s over a range of 100 mV vs. Saturated Calomel Electrodes (SCE) where Platinum foil (20 cm2) act as counter electrode. The open circuit potential was measured as V vs. SCE. |
Corrosion rate measurement Weight loss method
|
| The accurately weighed mild steel specimens (2 x 1 x 0.2 cm) were immersed in 500 mL of water for a period of 30 days. After 30 days of exposure in aqueous environment, the specimen were taken out cleaned by using pickling solution dried and weighed, corrosion rate is measured from the difference in weight of the specimen before and after immersion in water. The inhibition efficiency (IE) was calculate by using the formula |
| IE (%) = (corrosion rate without inhibitor - corrosion rate with inhibitor) X 100 Corrosion rate without inhibitor |
Polarization method
|
| The set up of polarization is already given in section 2.4. From the polarization graph, the corrosion current density (icorr) is calculated. The inhibitor efficiency (IE) is calculated using the equation |
| IE (%) = (icorr at blank inhibitor - icorr at inhibitor )X 100 icorr at blank inhibitor |
Corrosion at different duration of immersion
|
| The corrosion experiment set up is same as above (weight loss method). 1mL of electrolyte is taken from the corrosion cell at regular intervals of time. The iron present in the electrolyte was analyzed using AAS method. From the quantity of iron in the solution at definite intervals of time, the corrosion rate is calculated. |
Stimulated condition
|
| High temperature studies were carried out by placing the corrosion cell on a hot plate. The turbulence was made by stirring the solution by using a hot plate with magnetic stirrer, which rotates at a speed of 100 rpm. |
Composition of scale
|
| The metal constituents present on the scale formed on the surface of samples were analyzed by AAS. The carbon content present in the scale was estimated by using Leco- Carbon Analyzer. |
Results and Discussion
|
Weight loss methods
|
Stagnant condition and room temperature
|
| An ability of the inhibitor is determined by calculating the ratio of its efficiency to concentration in medium. Table 3 summarizes the weight loss experimental results conducted by immersing the mild steel coupons at 30oC for an exposure of 30 days with different inhibitor load. The OCP values shifted to more anodic region with increase in inhibitor concentration in the corrosion cell. The shift of OCP to anodic region shows the inhibition efficiency of the inhibitor to protect the metal in the electrolyte medium. The corrosion rate also decreases with increase of inhibitor in the electrolyte. The corrosion rate decreased to a value of 0.20 mpy when the system contains 500 ppm of the inhibitor. Increase of the inhibitor above this will not have any improvement in corrosion rate value. Hence 500 ppm can be selected as the optimum inhibitor concentration. |
Stimulated condition
|
| The corrosion rate of mild steel at different conditions (electrolyte at room temperature and at 80 oC with and without stirring) were evaluated and given in Table 4. The inhibitor shows very good inhibition properties to protect mild steel even under stimulated conditions. The corrosion rate shows a slight increase under stimulated conditions. This is due to more time required for adsorption of the inhibitor on the surface of mild steel under stimulated conditions. |
Electrochemical Methods
|
Polarization method
|
| The data obtained from potentiostatic polarizations experiments were given in Table 5. The corrosion potential (Ecorr) shifted to anodic region when the system contains corrosion inhibitors. The anodic potential shift shows the effectiveness of the inhibitor to protect the metal from corrosion. At stimulated conditions, the Ecorr value was found to be in cathodic regions compared to the corrosion system under room temperature. The polarization experiments were conducted in mild steel specimen just after 30 minutes of exposure in electrolyte medium. Since in stimulated conditions, more time is required for effective adsorption of the inhibitor, less anodic potential shift in Ecorr value for the metal compared to mild steel under room temperature and stagnant condition. The corrosion rate is high at elevated temperature and stirring conditions. But it was noticed that, even at high temperature and stirring conditions, the inhibitor loaded system is found to be more anodic compared to blank at stagnant conditions. The corrosion current density (icorr) decrease as the inhibitor concentrations increases. The polarization results show that the inhibitor is found to be a very effective for protecting the mild steel in potable water even under stimulated conditions. |
OCP Decay study
|
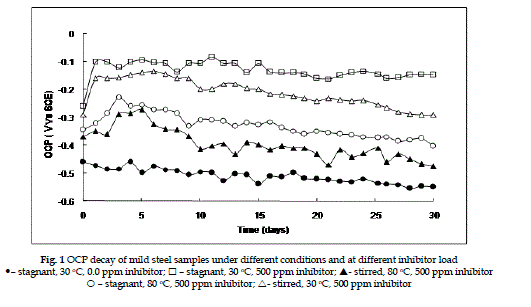
| Figure 1 shows the open circuit potential (OCP) decay of mild steel specimen in water with inhibitor at different conditions. The OCP of the mild steel in blank are also given for comparison. In all cases, the electrolyte with inhibitor, the OCP shifts to anodic regions and remains more or less constant for 30 days. The initial anodic shift of OCP is due to the adsorption of the inhibitor on the surface of the metal sample. The steady nature of OCP value for 30 days shows that the adsorbed film will remains well adhered on the metal surface even under stimulated conditions. |
Corrosion at different duration of immersion
|
| The corrosion rate was calculated for metal samples at different duration of immersion in electrolyte medium containing the inhibitor under different conditions. The corrosion rate calculation at different duration was carried out as explained in experimental section 2.5.3 and the corrosion rate is given in Table 6. |
| The corrosion rate decreased with increase in the period of immersion. As duration of immersion increases, the quantity of inhibitor adsorbed on the metal surface increases and there by decrease the exposure area of the metal. Hence the corrosion rate decreases with time. |
Composition of the scale
|
| The scale formed on the surface of the mild steel specimen immersed in electrolyte medium with inhibitor for 30 days of exposure was scratched out from the surface of the mild steel sample and analyzed for its constituents. The composition of the scale is given in Table 7. The calcium and magnesium present in the water make complexes with organic part present in the leaf extract (inhibitor). The complex will adhere on the surface of the mild steel and prevent the corrosion of the mild steel. |
Mechanism of Inhibition
|
| The OCP values of the mild steel sample becomes anodic when the electrolyte contains inhibitor showing that the inhibitor is adsorbed on the metal surface and prevent the for exposure of metal surface to the electrolytic medium. The corrosion rate decreases with increase in the time of immersion. As duration of immersion increases, the quantity of inhibitor adsorbed on the metal surface also increases. The carbon content present in the scale confirmed that an organic part of the leaf extract forms a film on the surface of the metal by adsorption process. This layer will adhere firmly and not depleted with time, temperature and agitated conditions. The formation of this layer is responsible for corrosion prevention of mild steel. |
Conclusion
|
| The asoka leaf extract is found to be good eco-friendly inhibitor to prevent the corrosion of mild steel in drinking water environment. The threshold concentration of the inhibitor was found to be 500 ppm. At stagnant condition, this concentration of inhibitor shows an inhibition efficiency of 92.4%. The inhibitor was found to be suitable for protecting the mild steel under stimulated conditions also. The inhibitor adsorbed on the surface of the metal and there by reducing the exposure of surface area of mild steel in the electrolytic medium. The quantity of inhibitor adsorbed increases with increase of time of exposure of mild steel in electrolyte. The organic part of the leaf extract is adsorbed on the surface of the metal and makes a complex on the surface of the metal. This layer will adhere firmly and not depleted with time, temperature and turbulence conditions. The formation of this layer is responsible for corrosion prevention of mild steel. |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
|
|
| |
Figures at a glance
|
 |
| Figure 1 |
|
| |
References
|
- Abd El-Rehim, S.S., Ibrahim, M.A.M. and Khaled, K.F.1999. 4-Aminoantipyrine as an inhibitor of mild steelcorrosion in HCl solution. Journal of Applied Electrochemistry29 : 593-599.
- Aiman El. Al-Rawajfeh and Ehab, M. Al-Shamaileh,2007. Inhibition of corrosion in steel water pipes byammonium pyrrolidinedithiocarbamate (APDTC).Desalination. 206 : 169 -178.
- Beenakumari, K.S. 2010. Development of environmentalfriendly inhibitor for corrosion prevention of mildsteel in aerated drinking water system. Anal. andBioanal. Electrochem. 2 : 36-40
- Beenakumari, K.S. 2011. Inhibitory effects of Murrayakoenigii (curry leaf) leaf extract on the corrosion of mild steel in 1 M HCl. Green Chemistry Letters and Reviews 4 : 117-120.
- Broo, A., Berghult, B. and Hedberg, T. 1999. Drinking water distribution - The effect of natural organic matter on the corrosion of iron and copper. Water Science and Technology. 40 : 17-24.
- Buchweishaija, J. and Mhinzi, G.S. 2008. Natural products as a source of environmentally friendly corrosion inhibitors: The case of gum exudate from acacia seyalvarseyal. PortugaliaeElectrochimicaActa. 26 : 257-265.
- Chauhan, L.R. and Gunasekaran, G. 2007. Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corrosion Science. 49 : 1143-1161.
- Olusegun, K. Abiola, J. and Otaigbe, O.E. 2009. The Effect of Phyllanthusamarus extract on corrosion and kinetics of corrosion process of aluminum in alkaline solution. Corrosion Science. 51 : 2790-2793.
- Orubite, O.K. and Oforka, N.C. 2004. Inhibition of the corrosion of mild steel in hydrochloric acid solution by the extract of leaves of nyapafruticans. Mater Lett. 58 1768-1772.
- Pradha, P., Joseph, L., Gupta, V., Chulet, R., Arya, H., Verma R. and Bajpai, A. 2009. Saracaasoca (Ashoka): A Review. J. Chemical and Pharaceutical Research 1: 62-71.
- Raja, P. B., and Sethuraman, M. 2008.Natural products as corrosion inhibitor for metals in corrosive media – A review. Material Letters. 62 : 113-116.
- Saha, J., Mitra, T., Gupta, K. and Mukergee, S. 2012. Phytoconstituents and HPTLC analysis in saracaasoka (ROXB) WILDE. International Journal of Pharmacy and Pharmaceutical Science. 4 : 96-99.
- Saratha, R., Priya, S.V. and Thilagavathy, P. 2009. Investigation of Citrus aurantiifolia leaves extract as corrosion inhibitor for mild steel in 1 M HCl. E-Journal of Chemistry. 6 : 785-795.
- Saratha, R. and Meenakshi, R. 2010. Corrosion inhibitor-A plant extract. Der PharmaChemica. 2 : 287-294.
- Sarojini, N. Manjari, S.A. and Chandrakanti, C. 2011. Phytochemical screening and antibacterial study of saracaindica leaves extract. International Research journal of Pharmacy. 2 : 176-179.
- Seetharam, Y. N., Sujeetha, H., Jyothishwaran, G., Barad, A., Sharanabasappa, G. and Shabana, P. 2003. Antibacterial activity of saracaasoca bark. Indian Journal of Pharmacy Science. 65 : 658-659.
- Sharma, S. K., Mudhoo, A., Jain, G. and Sharma, J. 2009. Inhibitory effects of Ocimumtenuiflorum (tulsi) on the corrosion of zinc in sulphuric acid: a Green approach. Rassayan. J. Chemistry. 2 : 332-339.
- Shams El din, A.M. 1986. The problem of ‘’red waters’’: A new approach to its solution. Desalination. 60 : 75-88.
- Smita, A.V. and Mehta, G.N. 1999. Effect of acid extracts of Acacia arabica on acid corrosion of mild steel. Bull Electrochem. 15 : 67-70.
|