Keywords
|
| Solar photofenton oxidation, Hydroxyl radicals, Biodegradability. |
INTRODUCTION
|
| Existing treatment system involves coagulation followed by the biological treatment for the latex wastewater. The industries adopt tricking filter, activated sludge process and oxidation pond as biological treatment system. Existing treatment methods for treating latex wastewater faced major drawbacks. Biological processes too have their limitation as it takes longer retention time for the degradation of pollutants and it has limitation with the degradation of non biodegradable organics present in the wastewater. Hence a high efficient and low cost treatment process, that could treat the latex wastewater is needed. |
| Photofenton oxidation is a process, which is based on the generation of hydroxyl radicals, which are highly reactive oxidants. The hydroxyl radicals have been extremely effective in the destruction of organic chemicals and they are non-selective. The hydroxyl radicals typically react one million to one billion times faster than chemical oxidants like ozone and hydrogen peroxide (Ghiziaine, 2003). The use of photofenton oxidation as a pretreatment step makes the wastewater more amenable for biotreatability (Sarria et al. 2002). Thus the photofenton process, one of the advanced oxidation processes (AOPs), has been widely applied for the treatment of industrial wastewaters from textile dyeing industry, distillery, chemical industry, kraft & pulp bleaching industry, etc. The photo-treatment goal is to remove biorecalcitrant compounds and total mineralization of pollutants is possible by the subsequent biological treatment step. Hence, a promising alternative to complete oxidation of biorecalcitrant wastewater is the use of Fenton oxidation as a pretreatment step to convert initial biorecalcitrant compounds into more readily biodegradable intermediates, which is to be followed by biological oxidation of these intermediates into biomass and water ( Sarria et al. 2003). |
| A coupled solar- photofenton and biological treatment system would be effective for the complete mineralization of biorecalcitrant pollutants and render the process cost effective. The overall performance of the coupled system is a function of the photochemical pretreatment time. Hence an optimal pretreatment time to stop the photo-treatment and switch over to the biological treatment is to be determined. The scope of the present study was to investigate the feasibility of applying coupled treatment of solar photofenton oxidation and biological process to the latex centrifuging effluent. |
MATERIALS AND METHODS
|
| Raw effluent was collected from a latex centrifuging industry located at Muppanthal village, Kanyakumari District, Tamilnadu, India and it was stored at 4°C by HAAKE EK 45 cooling system. Totally 10 number of samples were collected (2 samples everyday) in different occasions and were analysed for various physiochemical parameters as per APHA Standard Methods. The characteristics of raw effluent were pH (3.7-5.1), TSS (300-400 mg/L), TDS (23,000-28,000mg/L), BOD (3500-4200mg/L), COD (18,000-21,000 mg/L), Ammonia nitrogen (690-750 mg/L) &Total Kjeldahl nitrogen (840-910 mg/L). |
| The % purity of H2O2 was estimated by iodometric method. The molar ratio of fenton reagent was arrived as per the stoichiometric requirements with respect to COD (ie.1g COD =1g O2= 0.03125 mol of O2 = 0.0625 mol of H2O2 =2.125g of H2O2). The experimental set up consisted of 6 plexiglass beakers of size 0.17 x 0.17 x 0.18 (5L capacity each). Experiments were conducted using 1000 mL of wastewater for 90 minutes duration. Samples were collected at 30 minutes interval for analysis of COD reduction. For analysis of biodegradability study, experiments were conducted using 1000mL of wastewater for 6 h duration. Samples were collected at 1h interval for analysis of BOD and COD reduction. Losses of water by evaporation were evaluated in parallel experiment by measuring the solution in the reactor before and after the exposure of treament without water circulation to asses the effect of evaporation on biodegradability. |
| For biological study, a lab scale fully aerobic sequencing batch reactor (SBR) with an operating volume of 4L was used in the work. The phototreated wastewater was settled and neutralized by sodium hydroxide before feeding into the bioreactor. After acclimatization of biomass in the reactor, 1L of phototreated wastewater was fed to the reactor and treated by SBR cycles, Fill (0.5h), AERATE (5h), SETTLE (2h) and DECANT (0.5h). During the react phase, DO concentration of more than 2 mg/L was provided. Aeration and agitation of the reactor was ceased during the settle phase and sludge was allowed to settle under quiescent condition. The mixed liquor samples drawn from the SBR during its operation were analysed for pH, TSS, TDS, BOD, COD, NH4 –N and Kjeldahl nitrogen. The experiments were repeated by varying the MLSS concentration for two sets of wastewater that were given different contact time of photochemical treatment. |
RESULTS AND DISCUSSION
|
Pretreatment of solar photofenton process
|
| The enhancement of biodegradability of raw latex centrifuging wastewater using solar photofenton (Fe2+/H2O2 /solar) process was studied and effect of operational parameters such as pH, concentration of H2O2, dosage of Fe2+, liquid depth and contact time were studied. The further degradation of pollutants was also studied by sequencing batch reactor (SBR). Different batch runs were conducted with wastewater that was given two different photo-treatment time, so as to arrive at the optimum contact time. The effect of MLSS concentration was also studied. |
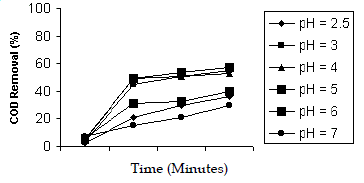
| The effect of pH was studied in the range of 2.5, 3, 4, 5, 6, 7 with H2O2/Fe2+ molar ratio 50:1. The maximum efficiency of COD reduction was observed in acidic pH of 3, 4, 5. This might due to the generation of Fe(OH)2+ which is generating the OH- in the pH of 2.5 (Rupert and Fallman, 1997) and hence the efficiency of COD reduction was more in the acidic pH. The efficiency decreased in pH 6 and above. This might be due to the formation of iron precipitates as hydroxide derivatives, reducing the Fe2+ availability and the radiation transmission, decomposition of H2O2 into water and oxygen (Sandra et al. 2001) and also due to the formation of hydroxyl radicals and increase in the quantity of unreacted H2O2 (Lidia et al. 2001). |
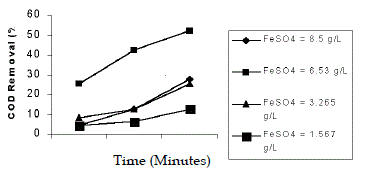
| The effect of dosage of Fe2+ was studied in the range of 65:1, 50:1, 25:1, 12:1. The pH was at 5 and the concentration of H2O2 was taken as 231mL/L. The maximum efficiency of COD reduction of 52% in 90 min was observed in 50:1, molar ratio (6.53 g/L). An increase in the concentration of Fe2+ did not improve the COD reduction and Fe2+ acts only as a catalyst. This was in conformity of the report (Chamarro et al. 2000). Agarwal et al. (2002) had shown that an increase in the amount of catalyst decreases the decolourisation. An increase in the concentration of Fe2+ did not improve the decolourisation (Josephine J. 2002). |
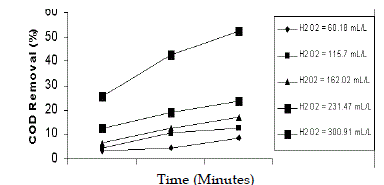
| The effect of concentration of H2O2 was studied for various molar ratios of 13:1, 25:1, 35:1, 50:1 and 65:1. The pH of wastewater was 5 and dosage of Fe2+ was 6.53 g/L. The maximum efficiency of COD reduction of 52% in 90 min was observed in 50:1 molar ratio (231 mL/L). The efficiency of COD removal was found to be less at 13:1 molar ratio, because enough quantity of H2O2 was not available and similar results were observed in the degradation of 4 - chlorophenol (Chammarro et al. 2001) and in the decolourisation of distillery effluent (Josephine,. 2002). Hung, et al. (2004) had shown that a perfect decomposition of phenol with finite time could be accomplished by the addition of optimum H2O2 regardless of irradiation. Miroslaw K (2004) concluded that fenton reaction depends mainly on hydrogen peroxide dose and ferrous ion ratio. |
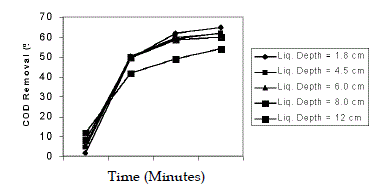
| Effect of liquid depth was studied in the range of 1.8, 4.5, 6, 8, 12 cm by varying the volume of wastewater from 500 mL to 3500 mL. The pH of wastewater was 5 and H2O2/Fe2+ molar ratio 50:1 was taken. The depth of wastewater upto 8 cm did not affect the efficiency of COD removal. This might be due to the intensity of light was not constant throughout the depth. This was in conformity of the earlier reports (Nappolian et al. 2000; Josephine, 2002). |
Effect of contact time on degradation/biodegradability
|
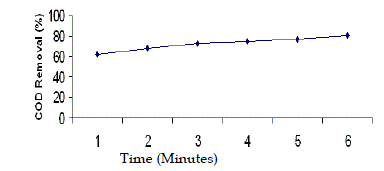
| In order to study the effect of contact time, biodegradability and evaporation loss, a parallel experiment was conducted for 6 hours duration. The pH of wastewater was 5 and H2O2/Fe2+ molar ratio was 50:1. Samples were collected at 1h, 2h, 3h, 4h, 5h, and 6h and analysed for BOD and COD. An intense oxidation was observed during the first 60 minutes of the process. With longer reaction time, there would not be more increase in BOD and COD reduction. A slow decrease of COD concentration was observed after 120 minutes. It was observed that BOD concentration was reduced from 3500 mg/L to 2300 mg/L, and COD concentration was reduced from 18,000 mg/L to 6000 mg/L during the first 2 hours irradiation. The BOD was further reduced from 2300 mg/L to 1770 mg/L and COD was further reduced from 6000 mg/L to 3600 mg/L during the latter 4 hours irradiation. Hence, the total BOD removal was found to be 50% and the total COD removal was found to be 81% during 6 hours irradiation. Similar results were observed in the treatment of textile dyeing effluent (Perez et al. 2002). Similar observation was also made in the treatment of distillery wastewater (Josephine, 2002). |
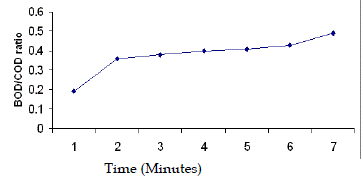
| Regarding Bio-degradability, it was observed that the BOD/COD ratios in the raw wastewater was quite low, ranging from 0.19 to 0.21. BOD/COD ratio was increased from 0.19 to 0.36 after 1 hour photochemical treatment. |
| This was in conformity of report of Sarria et al. (2000). He showed that the BOD/COD ratio of the phototreated effluent (AMBI) increased from 0.16 to 0.7 after photochemical oxidation. Isil A.B and Ferhan C. (1999) concluded that the photocatalytic oxidation led to an increase in the BOD5/COD of pulp bleaching wastewater. Ledakowicz S. and Solecka M. (2000) concluded that the applied chemical oxidation (AOPs) pretreatment of textile wastewater exerts a positive effect on its subsequent degradation. Evaporation losses after 6 hours ranged from 6-9%. Therefore results are not affected by this evaporation. BOD/COD ratio of 0.4 was achieved in an oxidation time of 3 hours. However 3 h and 5 h photochemical treatment time were selected for further study. The experimental results of solar photofenton treatment are presented in Fig. 1 to Fig. 6. |
Biotreatment of Phototreated Wastewater
|
| The fill and draw type of batch operation was carried out with phototreated wastewater (adjusted pH to 7). The sequencing batch reactor (SBR) system was initially fed with sewage and it was inoculated with activated sludge, operated aerobically. Then the reactor was slowly fed with 1 L phototreated latex wastewater (adjusted pH to 7) that was given 3 hours contact time of photochemical treatment, to make the microorganisms adopted to the new environment. Then the SBR was fed with 1 L of same phototreated wastewater (adjusted pH to 7). The retention time in each fill and draw run was 8 hours. The dissolved oxygen concentration in the reactor during the aerobic reaction was constantly above 2 mg/L which was adequate for microbial growth. Aeration was given continuously by compressor pump and mixing was given by mechanical stirrer. Several batch runs were conducted with wastewater that was given same photochemical treatment time and the effect of various MLSS concentration were studied. The successive repetition of a fill and draw operations were carried out with wastewater that was given 5 hours photochemical treatment time. The effect of various MLSS concentration was studied. The highest overall performance of combined treatment was obtained at 5 hrs photochemical treatment with MLSS concentration of 6560 mg/L in the bioreactor and the results are presented in Table 1. |
| The highest overall performance of combined treatment was observed with the wastewater that was given 5 hours photo-treatment time, with MLSS concentration of 6560 mg/L in the bioreactor. The removal efficiency of BOD 92%, COD 94%, TSS 84%, TDS 28%, Ammonia nitrogen 56% & TKN 58% were obtained. There was no significant difference observed with the wastewater that was given 3 hours phototreatment time, with MLSS concentration of 6620 mg/L in the bioreactor. The removal efficiency of BOD 92%, COD 93%, TSS 82%, TDS 35% Ammonia nitrogen 42% and TKN 45% were obtained. Hence, the optimal time to stop the phototreatment to shift the phototreated wastewater to a biological treatment system was observed as 3 hours. This was in conformity of the report of Sarria et al.(2000), who observed the shorter photochemical treatment time as the optimal phototreatment time for the degradation of the AMBI in the coupled system, by conducting different experiments, so as to render the process efficient and cost effective. Sandra et al. (2001) established 300 min as the optimal pretreatment time for the degradation of AMBI. His report showed that the pretreatment time should be short enough to make the coupled system cost effective. |
Conclusion
|
| Raw latex effluent had a low biodegradability (0.19 to 0.21) as determined from BOD5/COD ratios. The pretreatment of photofenton oxidation process led to an increase in biodegradability. The phototreated wastewater was found to be bio-compatible. As there are limitations in the treatment of latex wastewater by the existing treatment system, this combined solar photofenton and biological process could be used as an alternative technique, for the industries, especially located in the solar belt area. For the treatment of large quantities of latex wastewater, a pilot plant study for scaling up of the solar photofenton process need to be conducted to evaluate its applicability in the field. |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
 |
 |
 |
| Figure 4 |
Figure 5 |
Figure 6 |
|
| |
References
|
- Agarwal, Y.K., Pandya, B.R. and Menon, S.K. 2002. Kinetic studies on the Photocatalyticdecolourisation of water soluble crown dyes using Fe° &Zn catalyst. Indian Journal of Chemical Technology. 9 : 209-211.
- Chamarro, E., Marco, A. and Esplugas, S. 2000. Use of Fenton Reagant to improve organic chemical biodegradability. Water Research. 35 : 1047-1051.
- Ghiziaine, K., Nihal, O., Mehmat, O., Kacem, E.K. and Abderrahim, E.H. 2003. Degradation of herbicides by Fenton reaction. Environmental Chemistry, II:1-2.
- Hung, D.H., Sang, Y. and Hae, Y.Y. 2004. Improvement of oxidative decomposition of aqueous phenol by microwave irradiation in UV/H2O2 process and kinetic study. Journal of Water Research. 38 : 2782-2790.
- Isil, A.B. and Ferhan, C. 1999. Treatability of Kraft Pulp Bleaching wastewater by Bio-chemical photocatalytic oxidation. Jr. of Water Science Tech. 40 : 281-288.
- Josephine, J. 2002. Treatment of distillery effluent by solar photo-fenton oxidation process. CES, Anna University,
- Chennai. Leadakowicz, S. and Solecka M. 2000. Impact of advanced oxidation process on the biodegradation kinetics of Industrial wastewater. Water Science and Technology. 41: 157-164.
- Lidia, S., Claudia, J. Santosh, N.K. 2001. A comparative study on oxidation of disperse dyes by electrochemical process ozone, hypochlorite and fenton reagent. Wat. Res. 359 : 2129-2136.
- Miroslaw, K., Marcin, D., Adriana, D. and Marcin, Z. 2004. Chemical Oxygen Demand reduction of various wastewater types using magnetic field-assisted fenton reaction. Journal of Water Environment Research. 76 : 301-309.
- Neppolian, B., Sathivel, S., Banumathi, A., Palanichamy, M. and Murugesan, V. 2000. Zno-photo assisted degradation of textile dye using solar energy. Indian Journal Chemistry Technology. 8 : 36-40.
- Perez, M., Torrades, F., Domenech, X. and Peral, J. 2002. Fenton and photofenton oxidation of textile effluents. Water Research. 36 : 2703-2710.
- Rupert, B. and Fallman, H. 1997. The photofenton oxidation - A cheap and efficient wastewater treatment method. Res. chem. Intermed. 23 : 341-352.
- Sandra, P., Comninellis, C., Kiwi, J., Malato, S., Pichat, P. and Canonica, S. 2001. Coupling of photocatalytic and biological process as a contribution to the detoxification of water. International Ph.D Thesis.
- Sarria,V., Deront, M., Peringer, P. and Pulgarin, C. 2000. Degradation of biorecalcitrant dye precursor present in Industrial Wastewater by a new integrated Iron (III), photo assisted-biological treatment. Journal of Applied Catalysis B. Environmental 40 : 231-246.
|